닫기
카테고리버튼
pc gnb
Product Overview
Jinhaeng Waterway, a company that cares about customer health and nature's health
GT Ionizer
- Principle/effect
- Without external power supply, oxidation or reduction reaction occurs due to a potential difference
between metals. Zinc ions react with water and oxygen to generate hydrogen peroxide,
which decomposes organic matters and makes them precipitate.

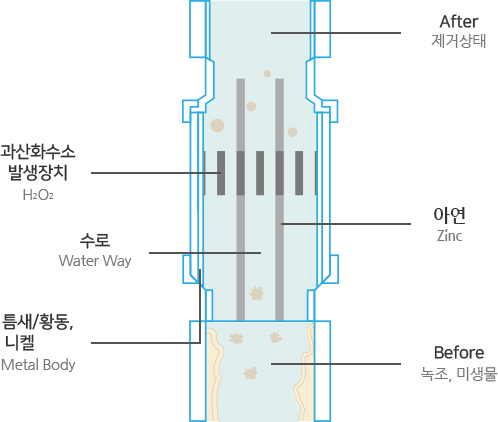
Structure Structure & Principles
Zn + 2H2O + O2 → Zn(OH)2 + H2O2
MechanismMechanism for removal of algal bloom
-
Step01
- Oxidation and reduction reactions generate oxidizing agents(hydrogen peroxide, H2O2)and produce OH radicals by the catalyzing action
of metal ions in water (Schumb ea al., 1955)
※ M is an unknown element. - Zn + 2H2O + O2 → Zn(OH)2 + H2O2 M2+ + 2H2O2 → M2+ + 2OH- + 2OH
- Oxidation and reduction reactions generate oxidizing agents(hydrogen peroxide, H2O2)and produce OH radicals by the catalyzing action
-
Step02
- Due to OH- generated, a pH value will be increased, and reactants with cohesive ability [Ca(OH)2] are produced. (White et al., 1998)
- M2+ + 2OH- → M(OH)2↓
-
Step03
- Ca(OH)2will react with phosphorus to produce Ca5(OH)(PO4)3and phosphate, an essential nutrient of green algae,
will precipitate → inhibiting the growth of green algae and abolishing green algae. - 20MO2 + 12PO43- + 2H2O → 4M5(PO4)3(OH)↓ + 19O2
- Ca(OH)2will react with phosphorus to produce Ca5(OH)(PO4)3and phosphate, an essential nutrient of green algae,
Optimization of system technology for removing algal bloom using zinc ionization REDOX
- TOP
- TOP